KNSB Alloy Propellant Development
5a. Melting of KNSB Alloys
Temperature and Slurry Characteristics
Objective
Procedure
Results
Discussion
Conclusions
Figures/Graphs
Objective
The purpose of this experiment is to compare the melting temperature and melted
slurry characteristics of several Potassium nitrate/Sorbitol (KNSB) formulas that are
alloyed with other sugars in varied amounts.
The % of KN is maintained at 65% The amounts of sorbitol and the alloy sugar are varied
with the total sugar (fuel) content maintained at 35%. (See table in Procedures below)
This experiment is a result of the Sugar Shot to Space Propellant Development Team's Proposed Investigation 5a
Be sure to check out the Sugar Shot to Space home page
Procedure
Materials:
The Potassium nitrate (KNO3) was fine powder (Stock #C170 - OX) from Firefox
The Sorbitol was Sorbo-Gem food grade powder. This grade of sorbitol is available from PVC ONLY
The Dextrose was anhydrous dextrose reagent grade
The Sucrose was ultrafine Baker's sugar C&H
The melting pot is a small triple batch double boiler utilizing paraffin as the heat transfer agent.
Thermometer is in centigrade, accurate to within 1 degree with 200C maximum
Process:
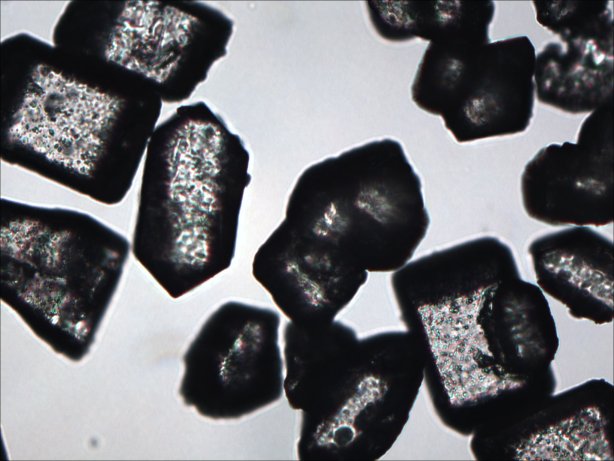
Grain size of the KNO3 can affect the pourability of the melted propellant so it was
examined in the microscope. Grain size range was measured with an ocular micrometer.
Since some of the sugars may not melt at lower temperatures their grain size was also examined.
Propellant batches were made in 20 grams total mass.
The ingredients for the alloy formula propellants in the table below were weighed
on a triple beam balance accurate to less than 0.1gm
| Designation |
Alloying sugar |
% Ratio 1: KNO3/Sorbitol/other |
% Ratio 2 |
% Ratio 3 |
% Ratio 4 |
| KNSBSU |
Sucrose |
65/30/5 |
65/25/10 |
65/20/15 |
65/15/20 |
| KNSBDX |
Dextrose |
65/30/5 |
65/25/10 |
65/20/15 |
65/15/20 |
As a non-alloy control plain KNSB 65/35 was also prepared.
The ingredients were then mixed by passing through a fine screen. This mixture
was then mixed multiple times by the "diaper method". This mixture was then placed
in sealed plastic containers and mixed further by vigorous shaking.
Each of the 20 gram batch formulas was then melted in a double boiler set up that
uses melted paraffin as the heat transfer medium. Figure 1 The double boiler set up prevents any chance of
an ignition hot spot in the melting process.
Initial attempts melted three propellants simultaneously using the paraffin temperature readings while recording consistency.
However this was problematic in that due to the small batch size the propellant could
be cooler than the paraffin. This problem was exaggerated with any breeze especially at the
higher melt temperatures. (Following and recording three propellant's change in consistency simultaneously was a bit tricky also.)
The process was started over with the thermometer inserted into a single propellant.
Figure 2
The oil bath was set to a temperature over the melting temperature of the higher melting
sugar.
The propellant was mixed with the thermometer as the stir stick and temperatures were recorded
as the propellant went through changes in it's melted state/consistency.
The readings are fairly subjective. The values are visual observations recorded on a scale of 1+ to 4+
1+ = scoopable,
2+ = very scoopable,
3+ = pourable, and
4+ = very pourable.
(If consistency is between very scoopable and pourable then
it is recorded as "2+-3+" or "2++") (2.5 for the graphs)
After recording consistency vs temperature the propellant was poured into 24mm "Bates" grains for the purpose of investigating cure rate (PDT - 5c)
See grains in Figure 3:
Figure 3
Results
Crystal size analysis:
KNO3 crystals:
The majority of the KNO3 crystals measured 20 microns to 200 microns
The smaller particles tended to stick to the larger ones

Sorbitol powder:
The majority of the sorbitol particles measured 10 to 200 microns

Anhydrous Dextrose crystals:
The majority of the dextrose crystals measured 20 to 300 microns

Sucrose:
The majority of the Sucrose crystals measured 100 to 300 microns
Melt Temperature/Consistency Data:
The following table lists the propellant alloy formulas (color coded)
succeeding rows show the temperature in Centigrade with the melted consistency graded on a 1+ to 4+ scale where:
1+ = scoopable,
2+ = very scoopable,
3+ = pourable, and
4+ = very pourable.
** = Starting to melt
PG = Poured Grain
TY = Trace of yellow degradation/caramelization; oil bath too high.
Y = Definite yellow degradation/caramelization; oil bath too high.
|
|
KNSB
|
|
KNSBSU
|
|
|
|
|
KNSBDX
|
|
|
|
|
|
65/35
|
|
65/30/5
|
65/25/10
|
65/20/15
|
65/15/20
|
|
65/30/5
|
65/25/10
|
65/20/15
|
65/15/20
|
|
90C
|
**
|
|
**
|
|
|
|
|
|
|
|
|
|
95
|
1+
|
|
|
**
|
|
|
|
|
|
|
|
|
97
|
|
|
1+
|
|
|
|
|
|
|
|
|
|
100
|
|
|
1+ - 2+
|
<1+
|
|
|
|
**
|
|
|
|
|
105
|
|
|
|
|
|
|
|
|
|
|
|
|
110
|
2+
|
|
|
|
|
|
|
1+
|
**
|
|
|
|
113
|
|
|
|
|
|
|
|
|
1+
|
|
|
|
115
|
2+-3+
|
|
|
|
|
|
|
|
|
|
|
|
117
|
|
|
2+
|
|
|
|
|
|
|
|
|
|
120
|
|
|
PG
|
1+
|
<1+
|
|
|
2+
|
2+
|
**
|
**
|
|
125
|
2+
|
|
|
|
|
|
|
|
|
2+
|
1+
|
|
127
|
|
|
|
|
1+
|
|
|
|
|
|
|
|
130
|
|
|
|
|
|
1+
|
|
2+ - 3+
|
2+ - 3+
|
|
2+
|
|
135
|
|
|
|
1+
|
2+
|
|
|
|
|
2+ - 3+
|
2+ - 3+
|
|
137
|
|
|
|
|
|
2+
|
|
|
|
|
|
|
140
|
2++
|
|
|
|
|
|
|
|
|
3+ PG
|
|
|
144
|
|
|
|
2+
|
|
|
|
|
|
|
|
|
145
|
|
|
|
|
|
2+ - 3+
|
|
2+ - 3+
|
|
|
2+-3+ Y
|
|
150
|
|
|
|
2++ TY
|
2+ - 3+
|
2+-3+TY
|
|
|
2+-3+TY
|
|
|
Discussion
The consistency readings are mostly subjective and based on small scoops of 1 to 4 grams. On a larger scale the readings would likely have been higher. The reasoning here is that, like honey, a small scoop will stick to the spoon (2+) whereas a larger mass will pour (3+)
Despite this variable of scale the readings are useful in comparing one formula to another since within this experiment the scale variable is controlled. Using the 1+ to 4+ scale was an attempt to make the data semi-objective. Looking at the data a pattern can be seen; with increasing dextrose or sucrose there is an increase the temperature at which initial melting occurs. The KNSB 65/35 is the control for this experiment. Those rocketeers familiar with this formula on a larger size scale can then compare it relative to the alloys.
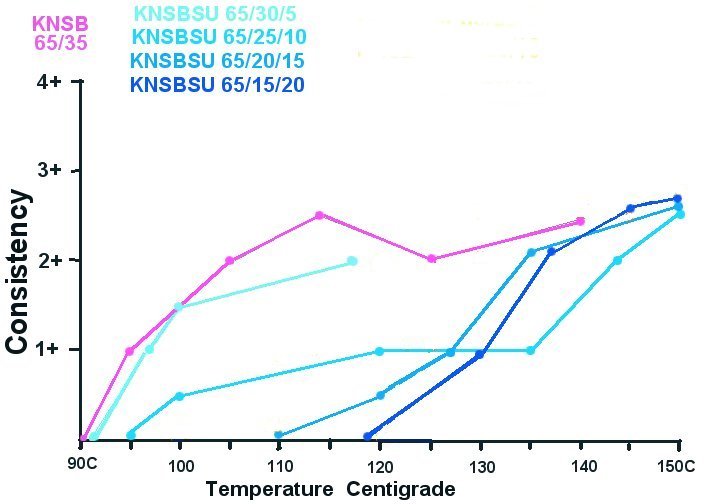
The 65/35 KNSB showed it's thinnest viscosity at approximately 115C which correlates to observations of others on SugPro of an optimum around 117C.
See Graph 1
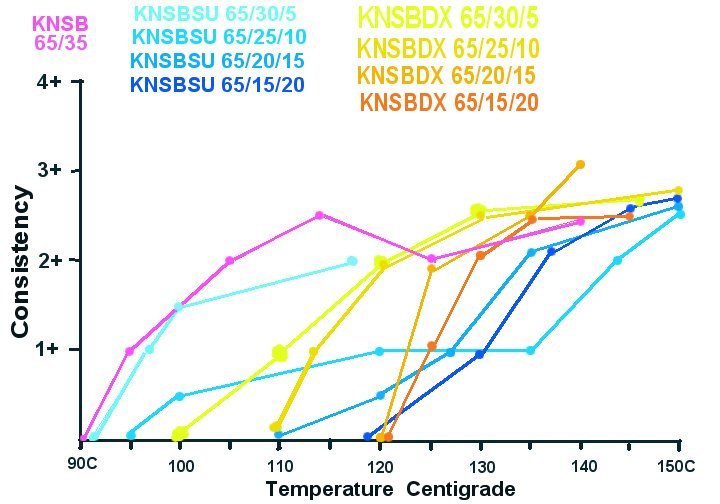
Graph 1 also shows the effect of increasing amounts of sucrose.
As expected the initial melting temperature increases with increasing sucrose. It is interesting that around 135 to 140C the higher % sucrose formulas appear to become less viscous.
This despite the fact that we have not yet reached the melting temperature of sucrose. (190C)
My hunch was perhaps the melted sorbitol was dissolving the sucrose.
Posing this question to the SugPro group brought a reply from Serge Pipko confirming this concept.
Cut and paste quote:
"Yes, they dissolve. This process takes some time, so the lower heating rate
you use, the lower complete melting temperature you will observe.
Serge"
end of cut and paste.
This experiment had quick melting of small amounts on fairly high heat so the increase in temperature was quite rapid.
The melting phenomena was not as obvious with the dextrose alloys. My experiment may not have provided sufficient time for anhydrous dextrose dissolving. However minimum viscosity was reached around 10 degrees less than the stated melt temp of anhydrous dextrose of 146C.
If dextrose "dissolving" is important to the propellant development than a slower/longer melting experiment should be attempted.
The dextrose consistency vs temperature data can be seen graphically in graph 2
Graph 2
The KNSBDX 65/20/15 formula did show the lowest viscosity of any formula (at 140C). The caveat here is the subjectivity of the 1-4+ readings. Also it is possible that particular reading was after a delay which could have allowed some of the "dissolving phenomena" to occur?
For comparing all dextrose alloy, sucrose alloy, and non-alloy control formulas see graph 3
Graph 3
Conclusions
This experiment provides preliminary data on the slurry characteristics of sucrose and dextrose alloys with sorbitol. There appears to be an advantage in that the alloys "melt/dissolve" at a lower temperature than the higher temp sugars would melt by themselves. This provides a buffer zone between the melt temperature and the temperature at which the dextrose or sucrose would caramelize.
However it should be noted that some caramelization was noticed at 150C for some sucrose alloys and 145-150C for some dextrose alloys. This is probably due to the fact that due to the small batches (on a cold windy day) the paraffin had to be heated noticeably hotter than the propellant temp. Though the propellant temps were 150C there may have been some higher heating of the thin layer of propellant directly against the metal bowl. Larger batches with more control on the oil temp would hopefully resolve this so that even 150C could have a good pot life.
If optimized viscosity becomes a high priority to the propellant developement than it would be good to narrow in on a few desirable alloys and do a larger and slower/longer melt experiment. Viscosity may be critical with some pouring methods, the auger concept comes to mind.
Should further analysis be a high priority then there could be ways to more objectively measure the slurry viscosity.
A couple ideas that come to mind are:
1. Melt a controlled measured depth of propellant and while it is still fully hot insert a heated large ball bearing. Measure the time it takes to sink a set amount.
2. Melt a controlled measured depth of propellant and while it is still fully hot insert a heated long metal rod that extends out above the propellant surface. Slant this rod a set number of degrees and measure it's rate of tipping over (angle change per time)
3. Melt a controlled mass of propellant and place it on a heated metal plate with an appropriate size hole. Measure the mass of propellant that falls/pours through over a set time.
Figures and Graphs
Figure 1:
Three formula set up

Figure 2:
Temperature recorded with thermometer as "stir stick"

Figure 3:
24mm Grains; Note yellowing on 20% dextrose formula.

Graph 1:
KNSB vs KNSBSU Alloys; Temperature vs Consistency:
Graph 2:
KNSB vs KNSBDX Alloys; Temperature vs Consistency:

Graph 3:
KNSB vs KNSBSU vs KNSBDX Alloys; Temperature vs Consistency: