The Potassium nitrate (KNO3) was fine powder (Stock #C170 - OX) from Firefox
The majority of the KNO3 crystals measured 20 microns to 200 microns
The smaller particles tended to stick to the larger ones

The Sorbitol was Sorbo-Gem food grade powder. This grade of sorbitol was available from PVC ONLY
The majority of the sorbitol particles measured 10 to 200 microns
The sorbitol will melt so crystal size is probably irrelevant to the results.

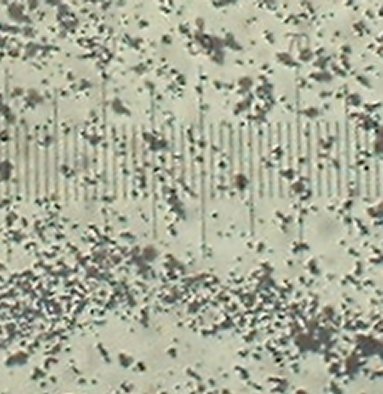
The TiO2 is very fine powder purchased at a rockshop. It is used by rockhounds as a rock polish.
The image below is at 400x and each line unit represents 2.2 microns.
Particles range from 0.5 microns to 2 microns. The objects that look larger are clumps of particles.
The fine particle size is for the purpose of giving rocks a smooth polished surface but this size is also the most efficient for reflecting light in the visible and infrared spectrum .
(500nm to 2,000nm particle size equates to the wavelengths of blue light up to the wavelengths of near infrared.)
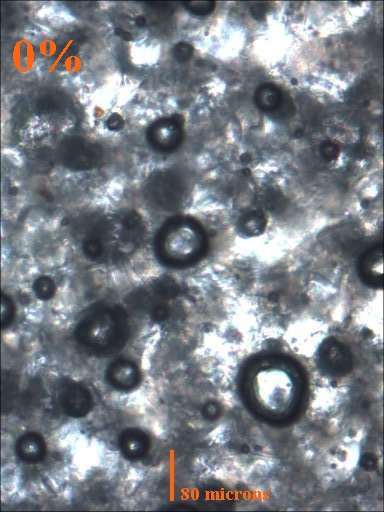
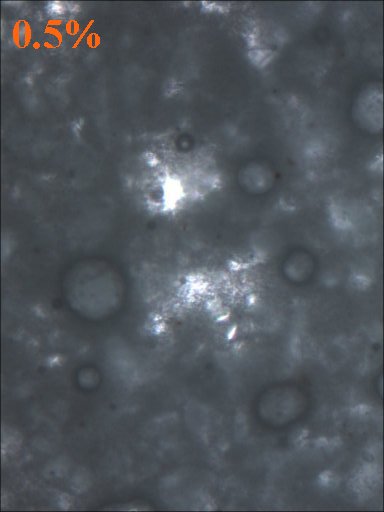
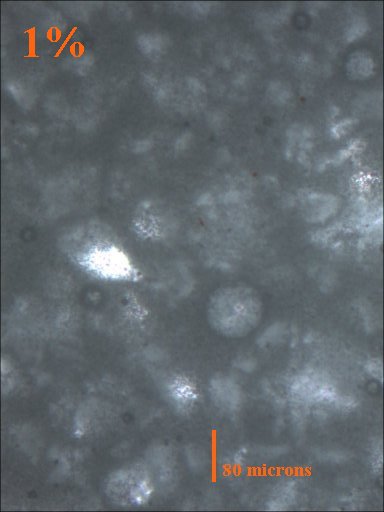
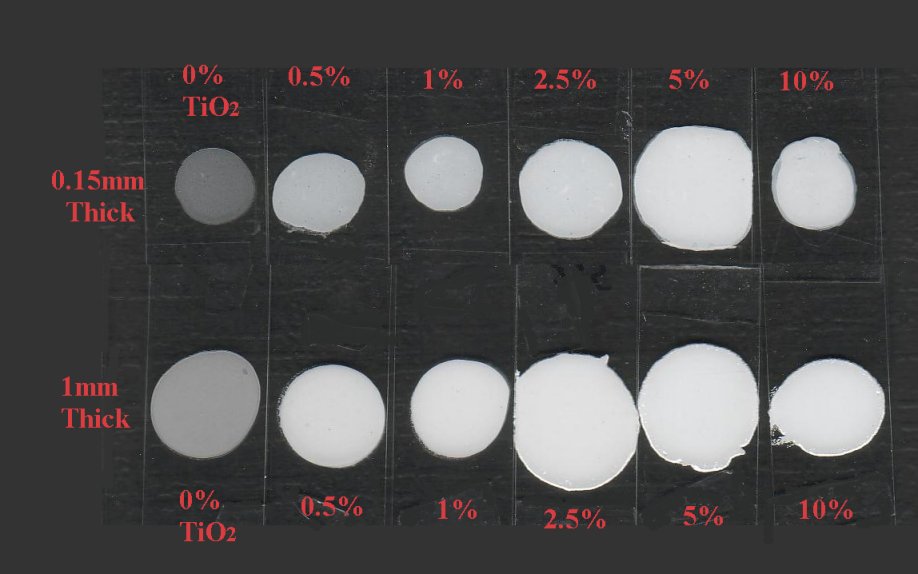
5 and 10 gram aliquots were taken from the initial 100 gram batch and TiO2 was added so that samples containing 10%, 5%, 2.5%, 1%, 0.5%, and 0% TiO2 were made.
Dry melting was performed with a wax double boiler.
10 gram samples melting:

5 gram samples melting:

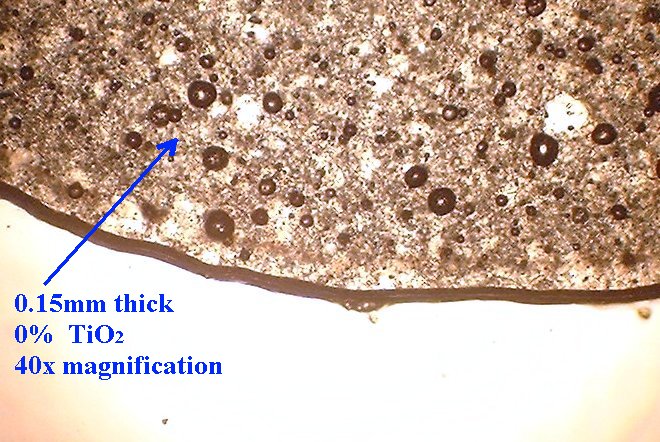
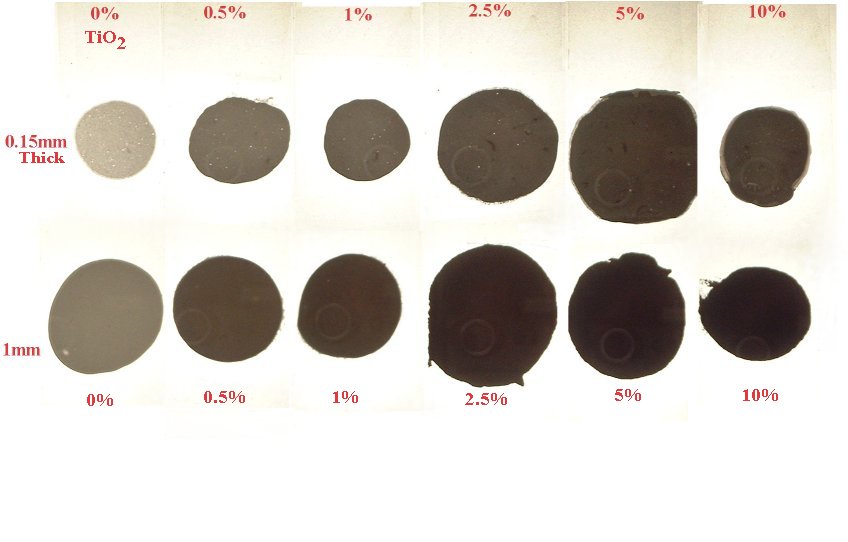
Specimens were pressed between microscope slides with spacers to make cured samples that were 0.15mm thick (150 microns) and another set that were 1mm thick (1000 microns)

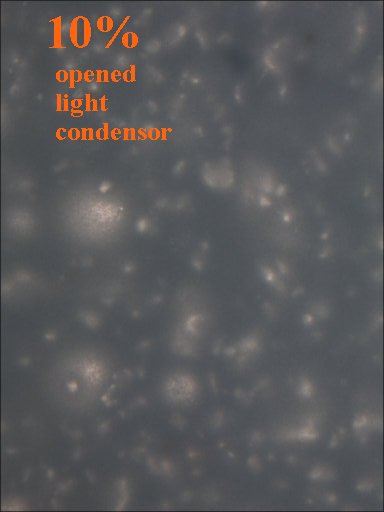
TiO2 powder is a strong reflector of infrared light and white light. Preliminary testing was performed with white light for simplicity and availablity of equipment.
Three methods for imaging:
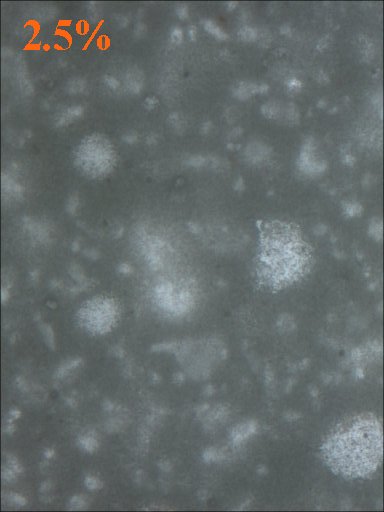
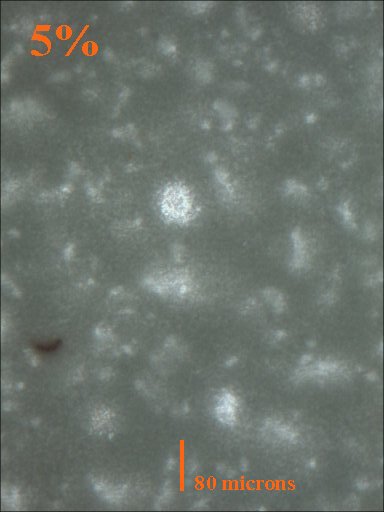
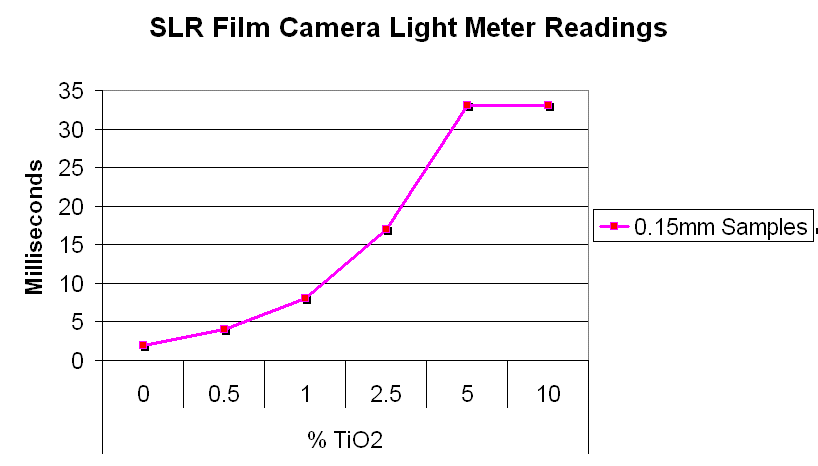
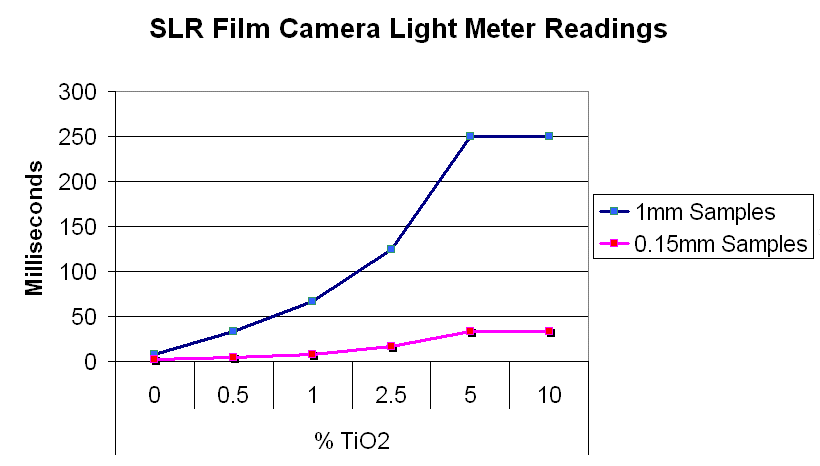
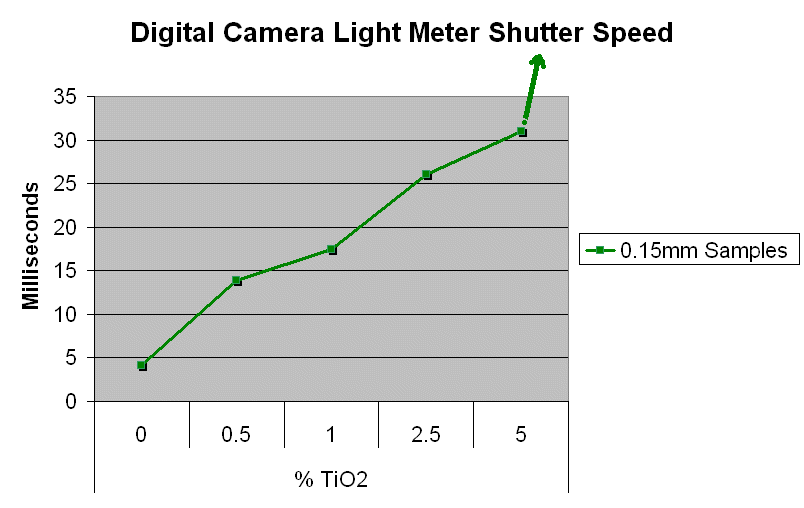
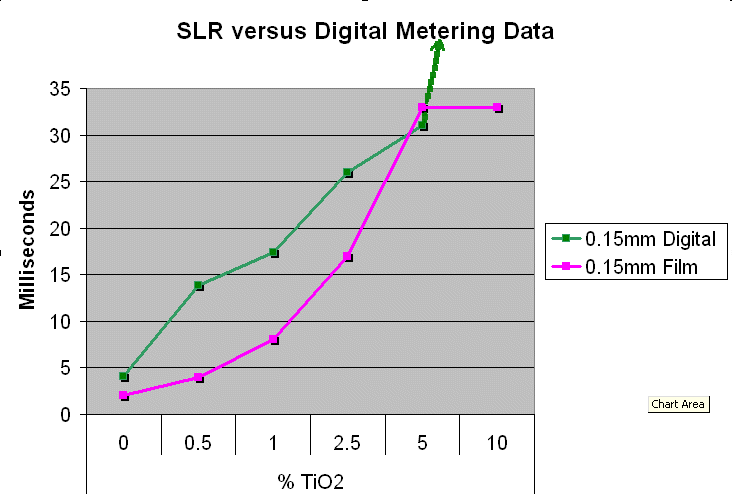
Method 1. The 0.15mm thick specimen slides were examined under a microscope at low power 40x with transmitted lightThis method allows for measurement of light penetration using the camera light meters resulting shutter settings
Method 2. The 0.15mm thick and 1 mm thick specimens were photographed macroscopically with transmitted light using a slide viewer.
Method 3. To capture data in an image for reflected/scattered light they were placed in a scanner with a black background and scanned.
To see what effect TiO2 has on burn rates the propellant was cast in two inch long strands and tested for burn rates at 1 atmosphere.